Various tumor-on-a-chip models have been developed so far but the architecture, composition and dialogue between cells still need to be more closely reproduced to obtain valuable answers to questions related to the spatio-temporal evolution of the tumor-microenvironment pair.
We have proposed a fabrication process to produce 3D fluidic devices with adapted aspect ratio for long term culture of tumor spheroids. 2PP lithography using the Nanoscribe system was applied for the fabrication of a master mold with different heights, for the fluidic adjacent channels and for the spheroid chamber, up to 500 µm. To ensure large-scale manufacture of series of fluidic devices at low cost, a very simple replication process in epoxy resist was also proposed to produce mold replica on large surface.
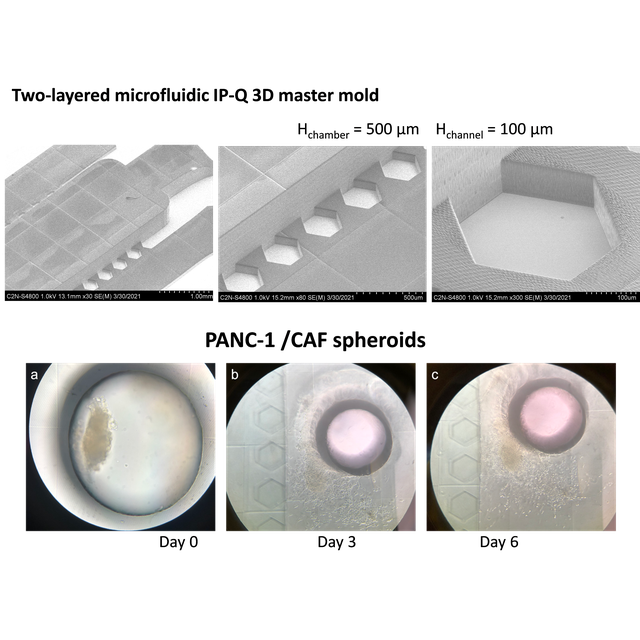
Then, heterotypic pancreatic cancer tumor spheroids, used as a model of a tumor with a strong fibrotic reaction, have been loaded and cultured in the device. If the height of the central chamber is too small and limited to 100 µm, the spheroid appears flattened and can only grow horizontally due to the restricted space available. Raising the height of the chamber to 500 µm promotes more gradual cell growth and migration, thanks to the availability of space for spheroid evolution. Thus, by adjusting the aspect ratio, we have designed and investigated different devices. And, we have provided a proof of concept of the opportunity for their application for tumor spheroids culture.
They might find potential application as a tool to gain in-depth knowledge of how the microenvironment (e.g., biochemical and biomechanical cues) governs tumor function (e.g., growth and migratory capacity) but also how cells affect their 3D environment (e.g., matrix degradation, creation of metabolic gradients).
References :
Fabrication of high aspect ratio microfluidic devices for long term in vitro culture of 3D tumor models
Martina Ugrinica,b, Dominique Decaninib, Nadège Bidana, Gianpiero Lazzaria, Abdelmounaim Harourib, Gilgueng Hwangb, Anne-Marie Haghiri-Gosnetb,*, Simona Muraa,*
Microelectronic Engineering 267–268 (2023) 111898
DOI : https://doi.org/10.1016/j.mee.2022.111898
This work has been supported by a public grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (reference: ANR-10-LABX-0035, Labex NanoSaclay)” and by the Fondation ARC pour la recherche sur le cancer (PJA20181207698).
aUniversité Paris-Saclay, CNRS, Institut Galien Paris-Saclay, 92296, Châtenay-Malabry, France
bCentre de Nanosciences et Nanotechnologies, UMR9001, CNRS, Université Paris-Saclay, 91120, Palaiseau, France
Figure : (a) Side view SEM images of two-layered microfluidic IP-Q 3D master mold with the 500 μm-high central chamber and the 100 μm-high lateral channels, and magnification on the pillar structure; (b) representative images of PANC1:CAF08 heterotypic spheroids cultured in the device over a period of 8 days. Day 0 indicates the day of spheroid transfer via the punched hole (diameter: 1 mm; black border inner ring in the images).